

An international research group has used theoretical predictions to identify and then fabricate a new electrocatalyst that significantly aids the oxygen evolution reaction (OER).
Details of their findings were published in the journal Advanced Materials on April 18, 2023.
Water-splitting electrolyzers harness electrolysis to uncouple hydrogen from oxygen. The generated hydrogen gas can be used as clean fuel whilst the oxygen gas can be utilised for medical purposes, industrial processes, or simply released into the atmosphere.
The OER, where water molecules are oxidized at the anode, has proven to be a barrier to these devices’ wider application. The OER causes energy loss and requires extra voltage to drive the reaction (overpotential).
To circumvent this, scientists have turned to rare-earth based transition metal oxides (RE–TMO), which helps achieve a more efficient OER process. But these are based on expensive and scarce metals, which further limits their industrial application. Moreover, much remains unknown about how they work.
“The predictive power of theory helped us overcome this shortcoming,” said Hao Li, associate professor at Tohoku University's Advanced Institute for Materials Research (WPI-AIMR) and corresponding author of the paper. “Given that finding effective and low-cost OER catalysts requires a lot of trial-and-error, we used theory to predict that doping cerium (Ce) into cobalt oxides (CoO) would lead to a better performing and more stable electrocatalyst.”
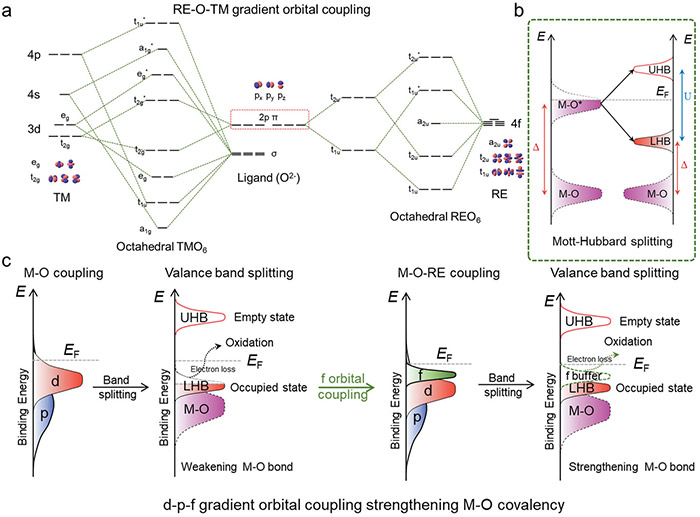
The proposed theoretical mechanism for the rationality in the gradient orbital coupling of TM–O–RE unit site. © Hao Li et al.
And the group’s predictions proved true. Using a special plasma technique, they combined cerium with the cobalt oxide before running tests on the material. The results confirmed the favorable performance, with an overpotential of only 261 mV at 10 mA cm−2 and robust electrochemical stability, superior to individual CoO.
X-ray absorption spectroscopy and in situ electrochemical Raman spectroscopy revealed that the cerium atoms made the cobalt oxide stronger by changing how the atoms are connected. The new arrangement of the atoms lead to a more efficient OER.
Li and his team are confident that their breakthrough can lead to further developments in creating more efficient RE–TMOs. “We believe our Ce-CoO model can serve as a basis for the mechanistic understanding and structural design of high-performance RE–TMO catalysts.”
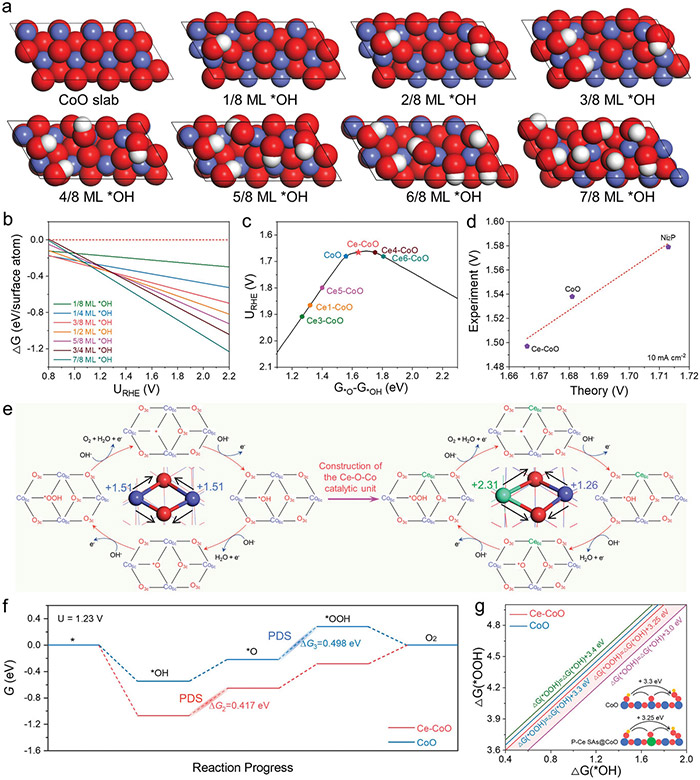
Theoretical analysis of OER activity and stability. © Hao Li et al.
| Title: | Reinforcing Co-O Covalency via Ce(4f)─O(2p)─Co(3d) Gradient Orbital Coupling for High-Efficiency Oxygen Evolution |
|---|---|
| Authors: | Meng Li, Xuan Wang, Kun Liu, Huamei Sun, Dongmei Sun, Kai Huang, Yawen Tang, Wei Xing, Hao Li* (Corresponding Author), Gengtao Fu* |
| Journal: | Advanced Materials |
| DOI: | 10.1002/adma.202302462 |
Hao Li
Advanced Institute for Materials Research (WPI-AIMR), Tohoku University
| E-mail: | li.hao.b8@tohoku.ac.jp |
|---|---|
| Webstie: | Hao Li Laboratory |